
In order to ensure customers satisfaction, continuous improvement of quality applied to the products and services is the priority of
Fin-ceramica Faenza S.p.A.
The quality- oriented policy of Fin-ceramica Faenza S.p.A. aims to involve all personnel since each person’s performance affects on the quality of devices manufactured and on the services offered.
Fin-ceramica Faenza S.p.A. has a Quality Management System in compliance with European Medical Device Regulation 2017/745, CFR 21 Part 820, GMP and is certified according to International Standard ISO 13485 and ISO 9001. The devices manufactured by Fin-Ceramica Faenza S.p.A. are certified according to European law. For the distribution of its products wordwide, Fin-ceramica Faenza S.p.A. applies local laws and specific requirements.

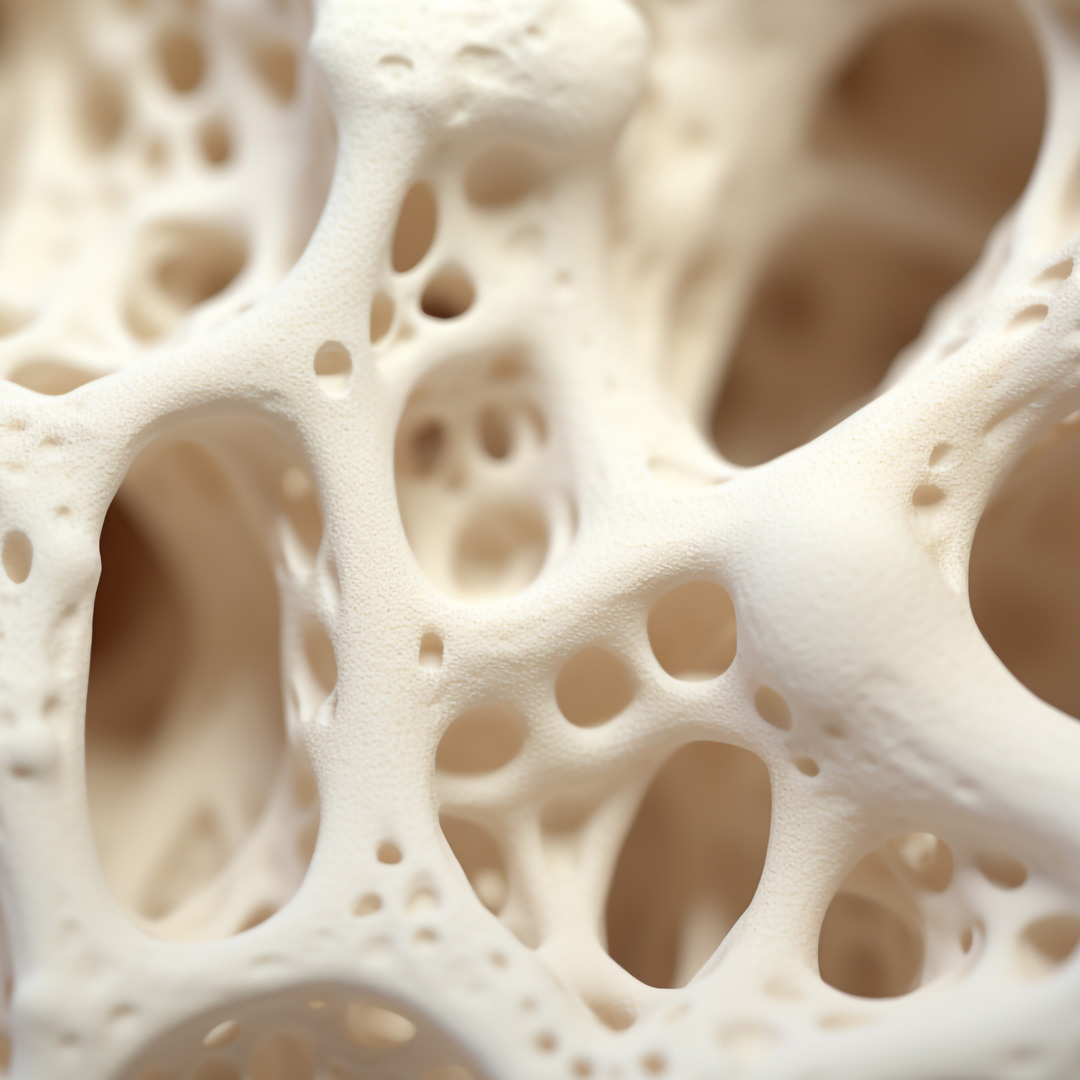
Customized Implant for Cranioplasty
Patient-specific cranial implants for advanced cranioplasty.
Explore resources by Product
CLINICAL STUDIES
Partners
Do you want to join our team?
Our challenge is to grow through new business partners who support us in the international distribution of our product lines.
Day by day, we strive to further develop a consistent and attentive relationship with our partners, based on listening and dialogue, analysis, and efficiency, to meet the needs of all our stakeholders.
Doctors, Hospitals and National Health Systems are the aim of our mission.
We are looking for partners who can help us improve the healthcare landscape through the reliable and innovative solutions we offer.
Why partner with Fin-ceramica S.p.A.?
Unique Portfolio
Gain access to an exclusive portfolio of medical devices that empowers you to serve a wide range of medical professionals.
Reliable and Safe
We are proud to have earned a great brand reputation synonymous with excellence, committed to ensure the highest standards of quality and safety.



 CustomizedBone
CustomizedBone