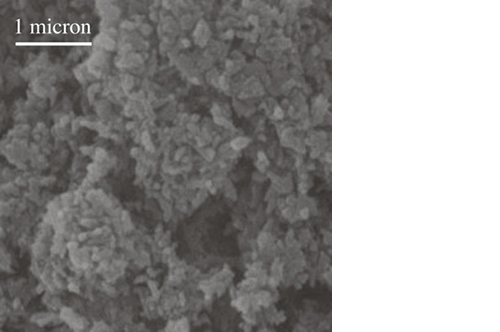
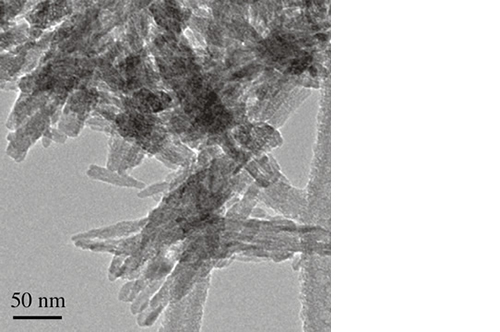
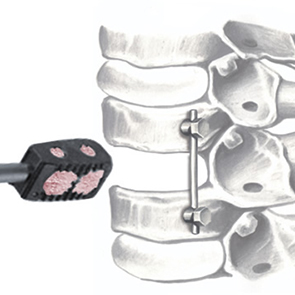
Mg-substituted apatite: just as nature designed it
It has been demonstrated that the presence of Mg2+ enables the hydroxyapatite crystal cell structure to become unstable and biologically active, promoting rapid cell-mediated material resorption, bone formation and remodelling.
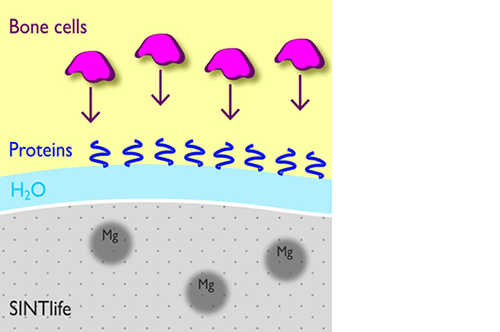
In addition, Mg2+ substituted apatite displays improved surface properties: the biomaterial effectively interacts with water molecules* to rapidly capture the key proteins involved in osteogenesis.
*Bertinetti et al. Langmuir (2009)
Synchronized process: material resorption and new bone formation
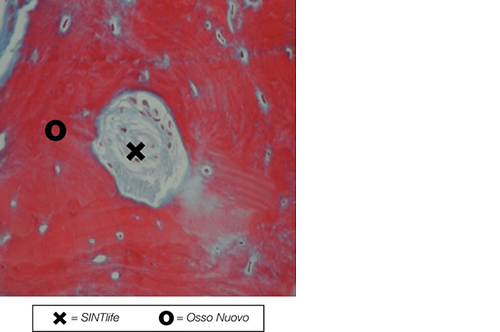
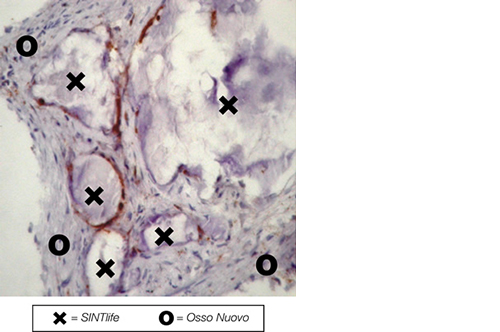
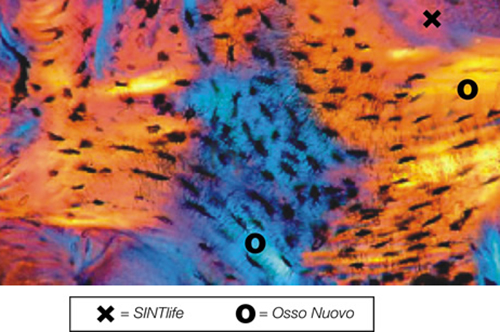
SINTlife is a unique material that interacts with bone-forming cells and promotes deposition of new bone tissue. Thanks to its specific biomimetic chemical composition, nano-structure and surface properties, SINTlife is remodelled and resorbed by cellular action over a period of 6-18 months, allowing for sufficient biostimulative scaffold to remain during the formation and maturation of new bone.
During the remodelling phase, osteoclastic resorption and osteoblastic osteogenenetic activities are seen around the remaining SINTlife particles*, up to a complete bone regeneration. As a result, SINTlife leads to a physiological, rapid and effective repair.
*Landi et al., J. Mater Sci: Mater Med (2008)