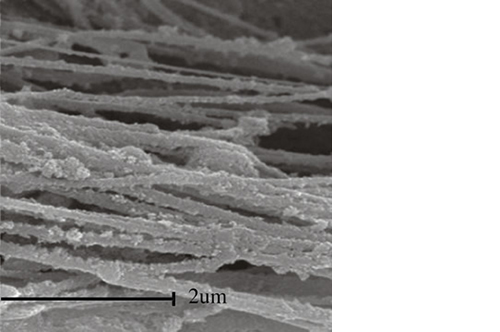
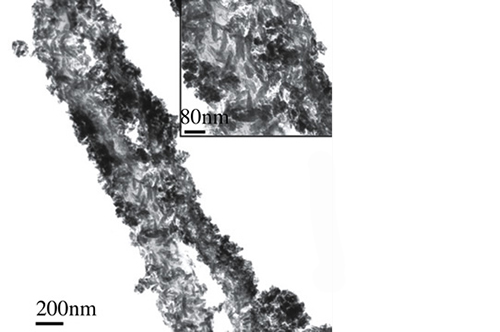
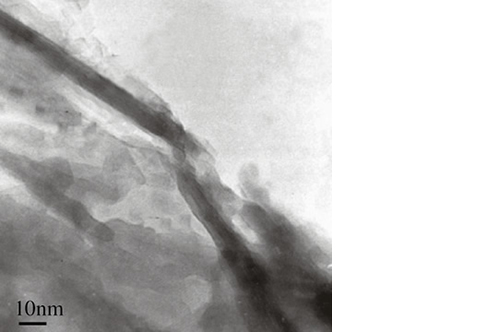
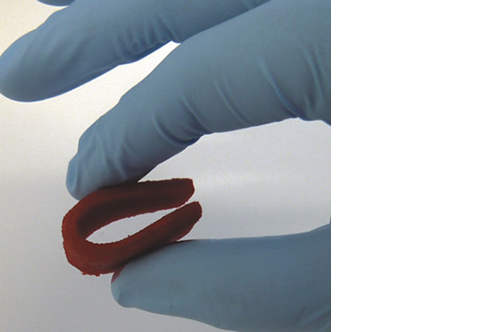
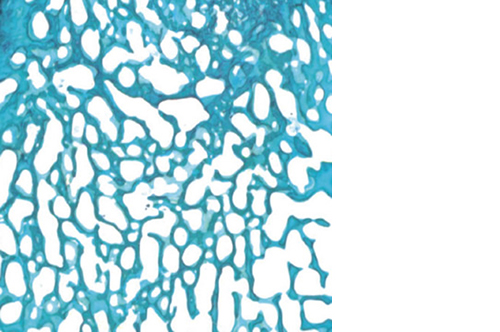
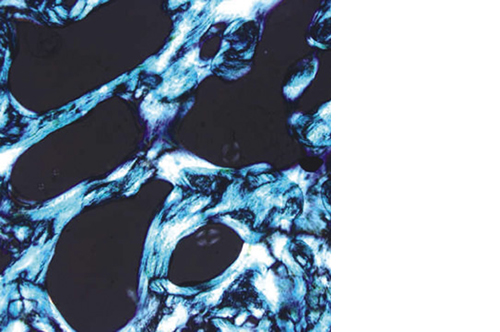
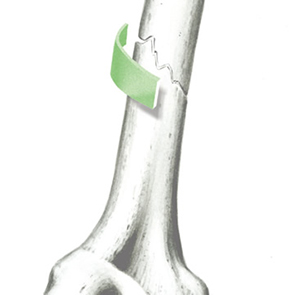
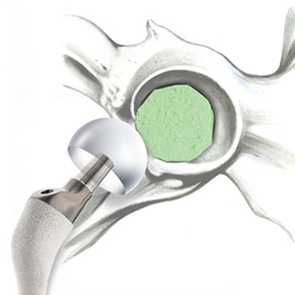
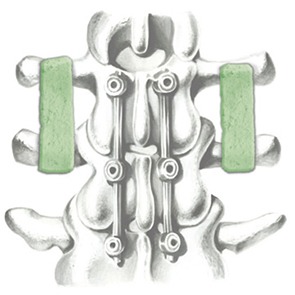
Biomimetic nano-structure: as modelled in nature
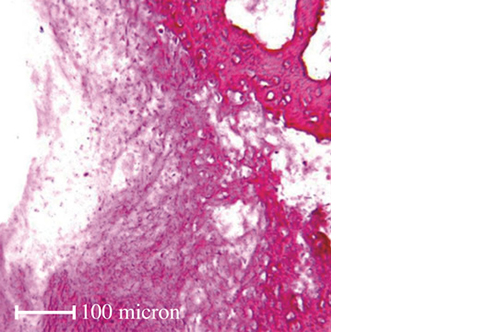
RegenOss is a fully biomimetic scaffold, its unique structure and chemical composition confer characteristics replicating that of human bone.
By using nature as a model, the patented nucleation of magnesium-enriched hydroxyapatite nano-crystals into type I collagen fibers resembles the process occurring during biological neo-ossification.