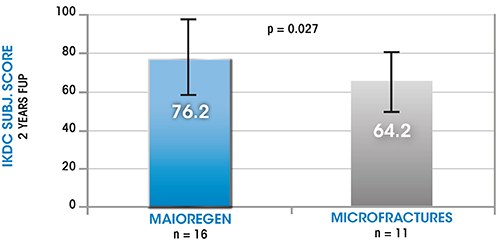
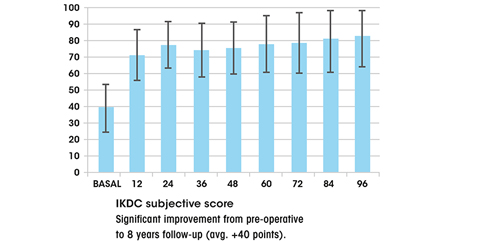
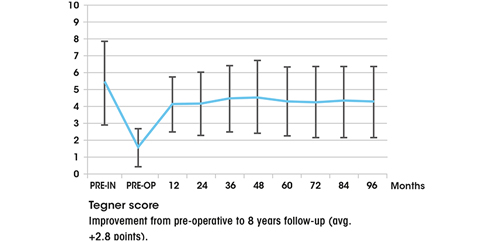
Composition and structure
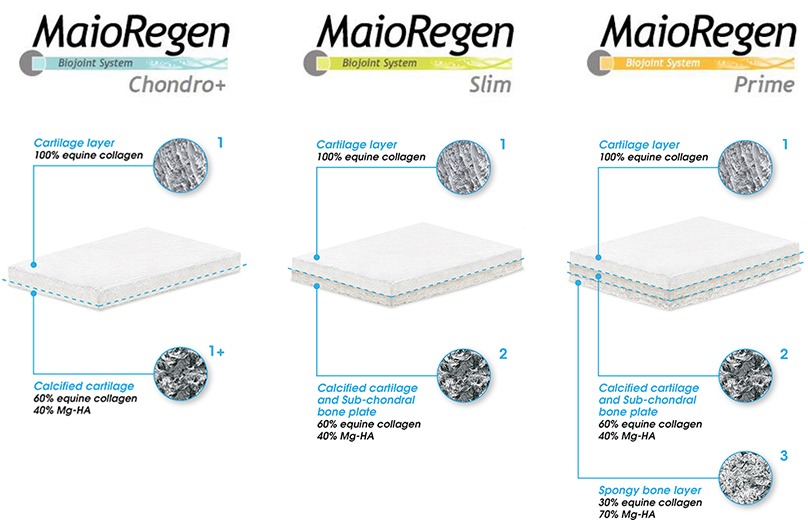
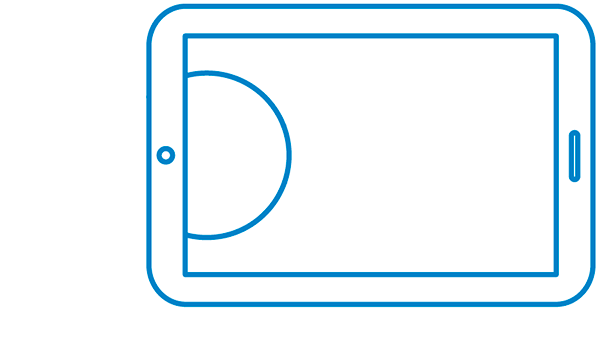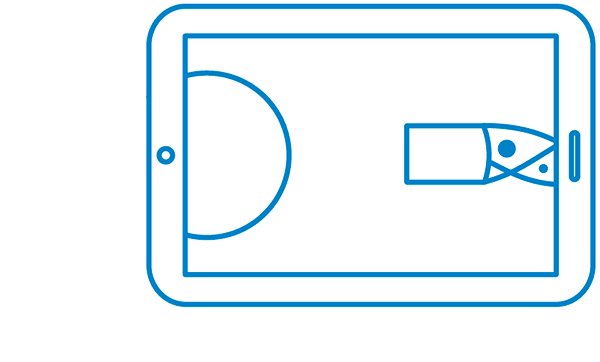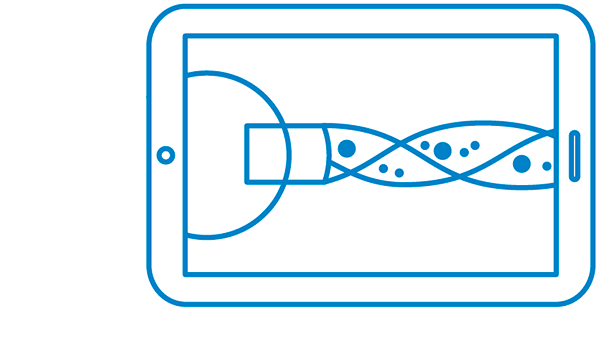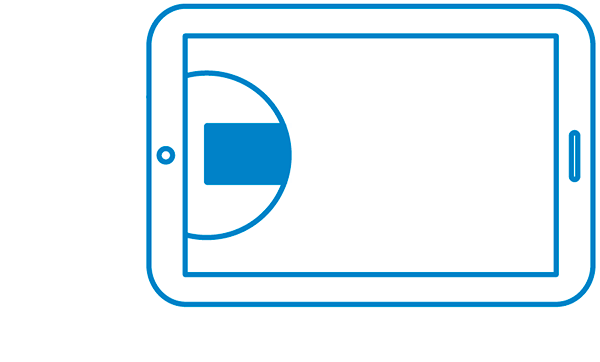
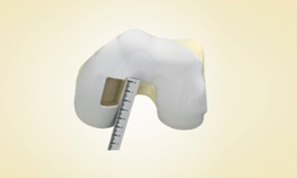
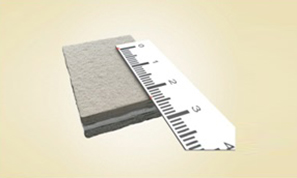
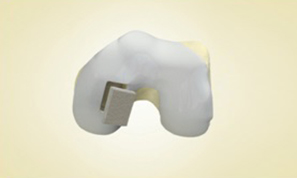
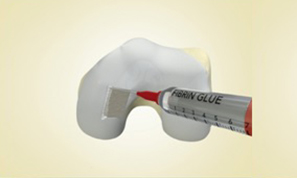
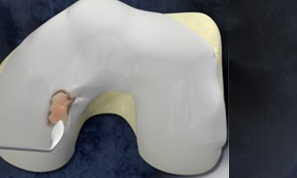
MaioRegen is a multi-layered matrix, manufactured through a patented process.
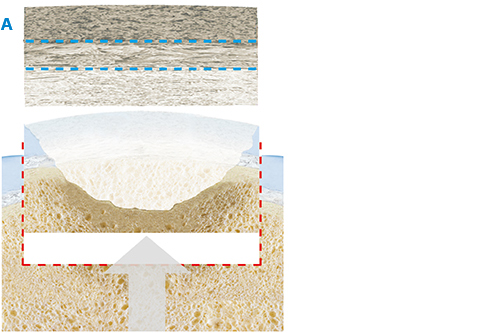
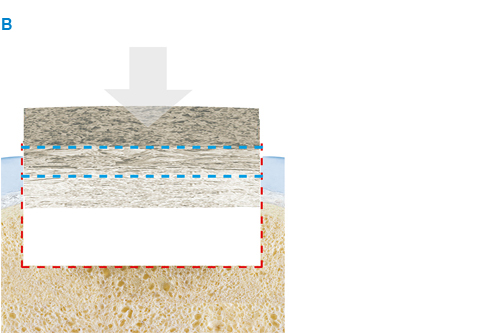
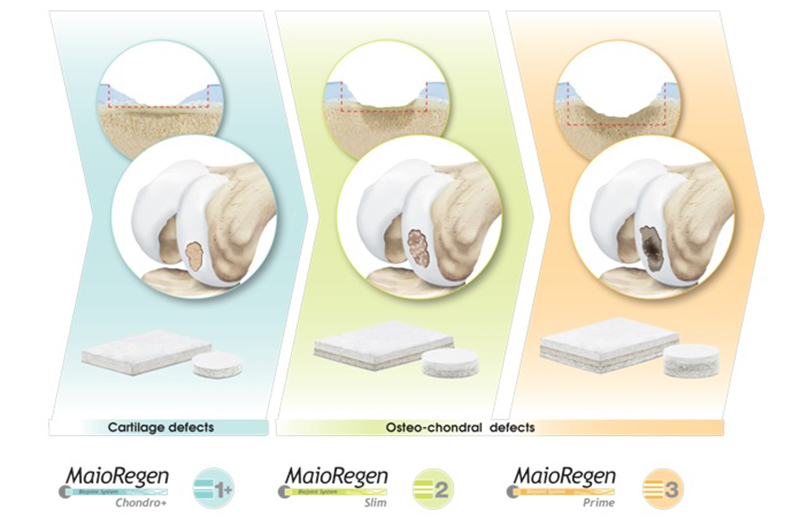
The product, composed by collagen and hydroxyapatite enriched with magnesium, mimics the chondral and osteochondral tissues, both in the chemical composition and in the micro- and nano-structure.
MaioRegen is available in three different configurations: MaioRegen Prime, MaioRegen Slim and MaioRegen Chondro+ represent specific solutions for the treatment of the different phases of early phases of arthritic pathology.