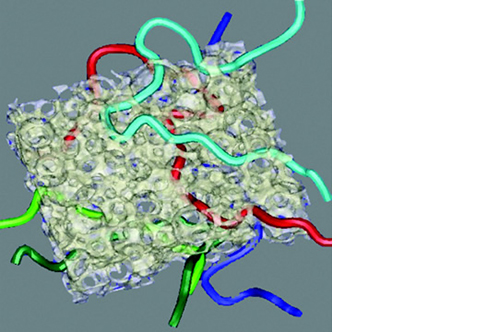
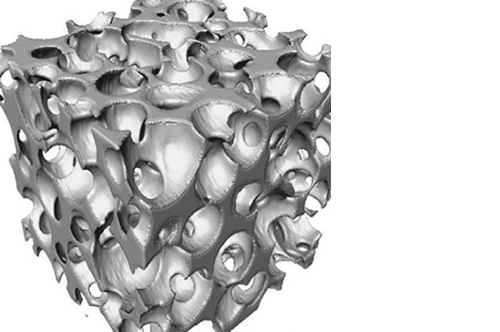
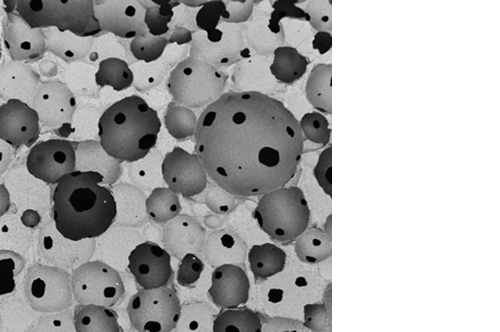
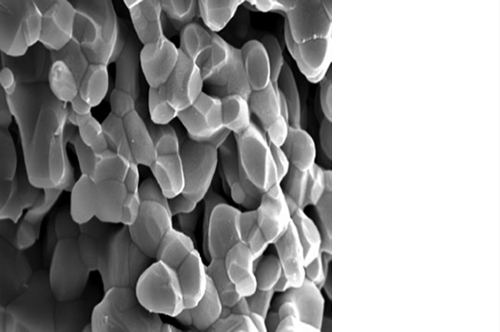
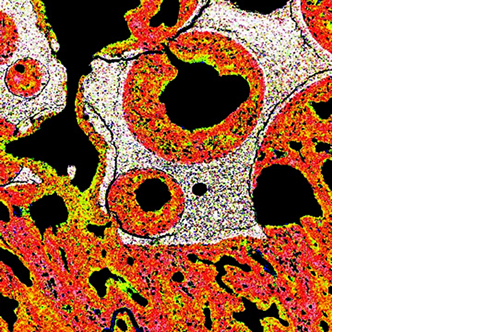
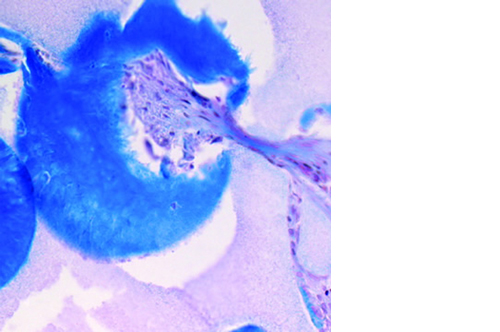
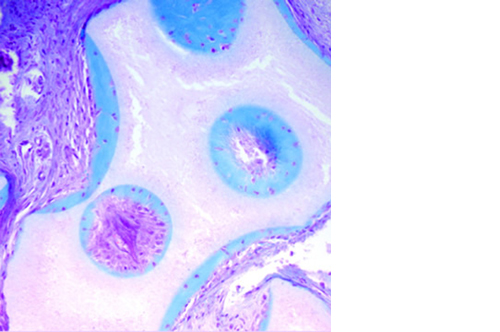
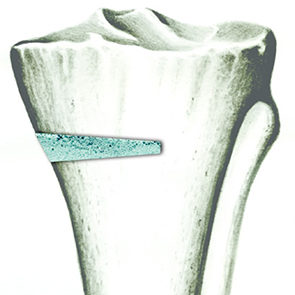
Micro, macro and interconnected porosity
This biomaterial has a unique, controlled micro and macro porosity along with an effective structure that supports rapid bone ingrowth and formation.
Its unique structure gives ENGIpore a porosity of almost 90%, and this guarantees an easy access of cells, biological fluids, and attracting the correct molecules throughout the bone substitute.
Despite the highly porous structure, ENGIpore is able to resist the compression forces associated with natural bone.